Ministry of Health has cleared eight private health facilities in Kampala to administer hepatitis B vaccination, as investigation into counterfeited vaccines continues, reports the Observer.
The private health facilities that have been cleared to provide the hepatitis B vaccine are: Nsambya hospital, Kibuli hospital, Mengo hospital, Rubaga hospital, International Hospital Kampala (IHK), Norvik hospital, Case hospital and Nakasero hospital.
National Drug Authority (NDA) during its routine post-marketing surveillance encountered falsified hepatitis B vaccines from eight health facilities in Mbarara, Mbale, Wakiso and Kampala districts.
The facilities are; Mbarara Community hospital, Divine Mercy, Mayanja Memorial, Family Doctors clinic Ntungamo, Mbarara City clinic, UMC hospital Bukoto, Malcolm Health care Kisaasi, and Kampala Medical Chambers on Buganda Road.
Hepatitis B vaccine is administered to healthy people to immunise them against the hepatitis B virus. According to minister of Health for General duties, Sarah Opendi, government has stopped all other private health facilities from administering the vaccine as investigations are going on.
“We shall advise the public on other facilities that have been cleared for hepatitis B vaccination in two weeks,” Opendi said in a statement.
District Health Officers (DHOs) and Resident District Commissioners (RDCs) have been instructed to ensure that the directive stopping private facilities from administering the vaccine is complied with. Immunisation of children as part of the routine practice will continue. The ministry has called on the general public to be vigilant.
According to the statement, there are a number of registered brands for hepatitis B vaccine for supply to Uganda from various manufacturers worldwide represented by local pharmaceutical companies.
These include Biological E represented by Gittoes, Glaxo SmithKline represented by Eris Ltd, Human Biologicals Institute represented by TATA (U), LG Life Sciences, Sanofi Pasteur & Pasteur Merieux represented by Laborex, Serum Institute of India represented by Norvik Enterprises, and Shantha Biotec represented by Laborex.
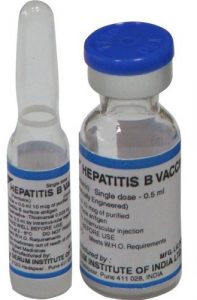
The brand that has been falsified is from Serum Institute of India. The vaccines from the company are available in two registered pack sizes i.e. one-millilitre vial and 10-millilitre multi-dose vials. The one ml vial is supplied to the private market and is identified by a white label with two green bands at the top and bottom part of the label, respectively.
The 10 ml multi-dose vial is supplied to the public (government) sector and is identified by a white label with two purple bands at the top and bottom part of the label, respectively. The vial supplied to Public Health Facilities also bears the words, “Government of Uganda, Not for sale”.
ALSO READ: National Drug Authority in the spotlight over negligence
Source: Observer Uganda